WHY IS DESIGN QUALIFICATION (DQ) ESSENTIAL WHEN INVESTING IN PHARMACEUTICAL MACHINERY?

✅ Verifies that the equipment design complies with GMP and international standards
✅ Ensures machinery is suitable for production, preventing unnecessary investment waste
✅ Reduces risks in later qualification stages (IQ, OQ, PQ)
✅ Avoids costly design errors and expensive modifications
✅ Supports the approval process for FDA, EMA, and Ministry of Health certifications
🚀 Without a proper DQ process, businesses may face technical errors, project delays, or even fail to obtain GMP certification!
📌 Invest right from the start – Don’t let design mistakes disrupt your entire production line!
READ MORE

Blister packaging plays a vital role in shielding active ingredients from moisture, oxygen, and light, ensuring product stability throughout its shelf life.

In the pharmaceutical industry, equipment must not only be modern — it must be validated to ensure safety, consistency, and GMP compliance.

In the pharmaceutical industry, choosing machinery is not just about price or technology—it must also meet strict quality standards. This is where URS (User Requirement Specification) plays a crucial role!

Choosing the right pharmaceutical manufacturing equipment is one of the critical factors determining the success of an investment project. However, not everyone understands and implements it correctly. Below are some guidelines to help you select pharmaceutical manufacturing equipment properly.

Choosing an unreliable supplier for pharmaceutical machinery can expose your business to serious risks

Quality and Reliability of Equipment
Ensure that machinery meets international certifications like CE and Atex, complies with GMP standards, and operates stably and durably. Quality management should follow ISO 9001.
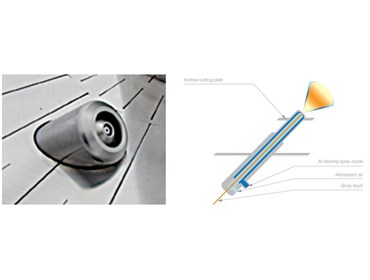
In modern pharmaceutical manufacturing, Tangential Spray technology in the Fluid Bed has brought a groundbreaking advancement. This advanced method involves spraying liquid materials (solutions or suspensions) at a tangential angle to the fluidized particles or pellets. The result? A swirling motion that ensures precise, uniform coating or granulation in a very short time.

The wall from ceiling down to the floor level, making the line fully visible from the operator side with no air ducts on the front side.

Ensuring operational safety in the pharmaceutical industry is critical to maintain product quality, protect employees, protect environment and comply with regulatory standards. Here are some key requirements for ensuring process machine operational safety in the pharmaceutical industry:
Selecting pharmaceutical machinery is a critical process that requires careful consideration to ensure compliance with regulatory requirements, product quality and operational efficiency.

In the ever-evolving landscape of pharmaceutical manufacturing, precision and efficiency take center stage. Our cutting-edge Auto Coating System revolutionizes the coating process, ensuring optimal product quality and streamlined operations. Let’s explore the key features that set our system apart:

Tien Tuan is thrilled to unveil our latest innovation in pharmaceutical containment technology – the Dispensing Isolator.

Some of challenges and typical benefits briefly of blister packing Anti-cancer tablet

Selecting pharmaceutical equipment is a critical process that requires careful consideration of various factors:

Child-resistant blister packaging is designed to prevent young children who may be curious and explore their environment from accessing and potentially ingesting harmful substances such as medications, yet remain relatively easy for adults to access.

FAT and GMP: IMPORTANT LINKS IN THE INVESTMENT PROJECT FOR PHARMACEUTICAL PLANT DEVELOPMENT


The wet granulation process in two separate steps is the most widely used technology and it is suitable for traditional drug mixing equipment such as Mixer & Granulator and Fluid-bed Dryer & Granulator.

The one-step granulation process helps to create granules directly, by granulating acid and alkali granules together. Usually, water is used as a granulation solution, so the effervescent reaction must be controlled during the granulation process. Ethanol or propanol can also be used as a granulation solution, but then additional binders are needed to achieve the necessary cohesion. There are 2 types of equipment used to prepare effervescent tablets according to this technology

Effervescent tablets have the main advantage of being easy to use, especially effective for patients who are children. However, their production is more complicated than traditional tablets, especially the selection of technology and equipment.
The effects of the new variant are difficult to announce accurately because unlike previous variants, Omicron mainly attacks young people who have better resistance.

Signing ceremony for cooperation in supplying fully automatic blistering cartoning line according to GMP-EU standard for US PHARMA

The Covid-19 pandemic has posed new challenges for pharmaceutical startups. What needs to be done to survive and develop, especially in this period. The StartUs Insight newspaper based on the analysis of “1745 famous pharmaceutical startups in 2020”, found the most outstanding startups. To turn challenges into opportunities, pharmaceutical companies have had “tips” for startups to survive the pandemic.


Checking the punches and dies and the components of the tablet press for wear or damage is one of the very important steps to improve the quality of the tablets. There are some points to check that we easily overlook but can cause significant quality problems for the tablets and reduce the life of the punches and dies. So take some time to check the following points to determine their wear or defects and quickly improve the quality of the tablets and reduce the cost of punches and dies

If you are interested in beauty, you must have heard of collagen skin care. Even many cosmetic companies have launched products that contain collagen, such as moisturizers, lotions, serums, etc.

Ở một số nơi, khi số ca mắc #COVID-19 giảm xuống, một số biện pháp phòng chống dịch đang được gỡ bỏ...

Ngành công nghiệp dược phẩm tuân theo các yêu cầu khắt khe nhất đối với quá trình sản xuất và thiết bị được sử dụng phải tuân thủ thực hành sản xuất tốt (GMP). Khi xu hướng phát triển thuốc thay đổi, các quy trình sản xuất cũng phải thay đổi, phần lớn trong số đó hiện có thể được tự động hóa...

Cuộc khủng hoảng y tế COVID-19 mang lại những tác động và tiến bộ khoa học mạnh mẽ đồng thời cũng rút ra được những bài học kinh nghiệm quý báu cho các doanh nghiệp, tổ chức và các quốc gia trên thế giới:
Tại Trung tâm chống dộc Bệnh viện Bạch Mai đã tiếp nhận rất nhiều trường hợp bị ngộ độc do lạm dụng thuốc hạ sốt paracetamol để chữa COVID-19. Do đó, người dân nên cẩn trọng trước các bài thuốc lan truyền không rõ nguồn gốc trên mạng xã hội, trước khi sử dụng bất kỳ loại thuốc nào cần xin ý kiến bác sĩ, không được tự ý sử dụng.

Từ ngày 1/8/2021 doanh nghiệp có thể tra cứu trạng thái chứng nhận xuất xứ mẫu D trên internet. Đồng thời các doanh nghiệp nhập khẩu các lô hàng thuốc, nguyên liệu làm thuốc nhập khẩu luồng đỏ phải xuất trình bản gốc giấy uỷ quyền, giấy phép lưu hành và phiếu kiểm nghiệm sản phẩm

Hiệp định thương mại tự do Việt Nam - EU (EVFTA) có hiệu lực từ tháng 8/2020 mở ra nhiều cơ hội trong quan hệ đối tác toàn diện giữa hai bên. Tác động của EVFTA đến ngành dược phẩm đã có những kết quả dần rõ nét, đặc biệt là đối với các doanh nghiệp Châu Âu.

Việc duy trì sản xuất và giao hàng đúng hạn, giữ đúng cam kết với khách hàng là nỗ lực đáng ghi nhận, thể hiện uy tín, nội lực và sức cạnh tranh cao của Tiến Tuấn.

Theo Đại sứ quán Việt Nam tại Ấn Độ, đây là gợi ý được các ông lớn trong ngành dược phẩm Ấn Độ đưa ra trong buổi họp xúc tiến thương mại đầu tư do Đại sứ Việt Nam tại Ấn Độ - ông Phạm Sanh Châu chủ trì.



